A spray that feels like a lotion
Want the lowest possible price on your Sernivo prescription?*
*Terms and conditions may apply.

*Terms and conditions may apply.

For hard-to-reach and wide-spread areas.
A thin, consistent, easy spray application.3

A lotion-like feel.
Because of its unique formulation once sprayed on the skin, it can be rubbed in like a lotion.2,4

Approved for use up to 4 weeks2

The most common side effects
of Sernivo Spray include itching, burning, stinging, pain, and thinning of skin (atrophy) at the treatment site. These are not all the possible side effects of Sernivo Spray.2 Please click to download full Prescribing Information.
References:
1. Wozel G. Psoriasis treatment in difficult locations: scalp, nail, and intertriginous areas. Clin Dermatol. Sep-Oct 2008;26(5):448-59.
2. Sernivo [package insert] Primus Pharmaceuticals, Inc. 2021.
3. Gold LS, Jackson JM, Knuckles MLF, Weiss JS. J Drugs Dermatol. 2016;15(3):334-342
4. Jackson J, Grove G, Allenby K, Houser T. DFD-01 Reduces Transepidermal Water Loss and Improves Skin Hydratin and Flexibility. Dermatol Ther (Heidelb) 2017;7:507-514
Click to get information TAILORED TO YOUR NEEDS

Putting the needs of PATIENTS FIRST
Primus makes it easier to prescribe, reducing burden for the patient and the physician in getting access to much-needed treatments.
LEARN MORE
SERNIVO® (betamethasone dipropionate) Spray, 0.05% for topical use
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
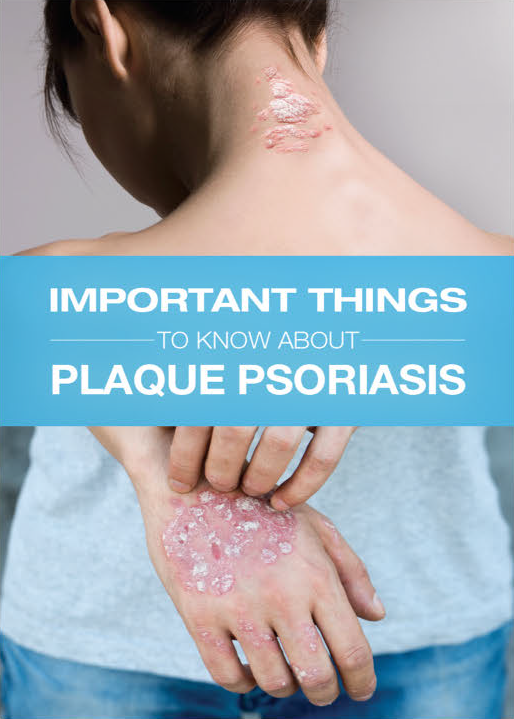
SERNIVO Spray is a corticosteroid indicated for the treatment of mild to moderate plaque psoriasis in patients 18 years of age and older.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Effects on Endocrine System: SERNIVO Spray can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency during or after treatment. Factors that predispose to HPA axis suppression include the use of high-potency corticosteroids, large treatment surface areas, prolonged use, use of occlusive dressings, altered skin barrier, liver failure, and young age. Evaluation for HPA axis suppression may be done by using the adrenocorticotropic hormone (ACTH) stimulation test. If HPA axis suppression is documented, gradually withdraw the drug, reduce the frequency of application, or substitute with a less potent corticosteroid. Systemic effects of topical corticosteroids may also manifest as Cushing's syndrome, hyperglycemia, and glucosuria. These events are rare and generally occur after prolonged exposure to larger than recommended doses, particularly with high-potency topical corticosteroids.
Ophthalmic Adverse Reactions: Topical corticosteroids may increase the risk of posterior subcapsular cataracts and glaucoma. Avoid contact of SERNIVO Spray with eyes. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
Allergic Contact Dermatitis: Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation. Corroborate such an observation with appropriate diagnostic patch testing. If irritation develops, discontinue the topical corticosteroid and institute appropriate therapy.
DOSAGE AND ADMINISTRATION
ADVERSE REACTIONS
The most common adverse reactions (≥ 1%) are application site reactions, including pruritis, burning and/or stinging, pain, and atrophy.
To report SUSPECTED ADVERSE REACTIONS, contact Primus Pharmaceuticals, Inc. at 1-480-483-1410 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
SERNIVO Spray is a corticosteroid indicated for the treatment of mild to moderate plaque psoriasis in patients 18 years of age and older.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Effects on Endocrine System: SERNIVO Spray can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency during or after treatment. Factors that predispose to HPA axis suppression include the use of high-potency corticosteroids, large treatment surface areas, prolonged use, use of occlusive dressings, altered skin barrier, liver failure, and young age. Evaluation for HPA axis suppression may be done by using the adrenocorticotropic hormone (ACTH) stimulation test. If HPA axis suppression is documented, gradually withdraw the drug, reduce the frequency of application, or substitute with a less potent corticosteroid. Systemic effects of topical corticosteroids may also manifest as Cushing's syndrome, hyperglycemia, and glucosuria. These events are rare and generally occur after prolonged exposure to larger than recommended doses, particularly with high-potency topical corticosteroids.
Ophthalmic Adverse Reactions: Topical corticosteroids may increase the risk of posterior subcapsular cataracts and glaucoma. Avoid contact of SERNIVO Spray with eyes. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
Allergic Contact Dermatitis: Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation. Corroborate such an observation with appropriate diagnostic patch testing. If irritation develops, discontinue the topical corticosteroid and institute appropriate therapy.
DOSAGE AND ADMINISTRATION
- Shake well before use.
- Apply SERNIVO Spray to the affected skin areas twice daily and rub in gently.
- Use SERNIVO Spray for up to 4 weeks of treatment. Treatment beyond 4 weeks is not recommended.
- Discontinue SERNIVO Spray when control is achieved.
- Do not use if atrophy is present at the treatment site.
- Do not bandage, cover, or wrap the treated skin area unless directed by a physician.
- Avoid use on the face, scalp, axilla, groin, or other intertriginous areas.
- SERNIVO Spray is for topical use only. It is not for oral, ophthalmic, or intravaginal use.
ADVERSE REACTIONS
The most common adverse reactions (≥ 1%) are application site reactions, including pruritis, burning and/or stinging, pain, and atrophy.
To report SUSPECTED ADVERSE REACTIONS, contact Primus Pharmaceuticals, Inc. at 1-480-483-1410 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.